Guangzhou Jet Bio-Filtration Co., Ltd. (stock code: 688026), founded in 2001 and located in Huangpu District of Guangzhou City, is a domestic leading new high-tech enterprise that offers total solutions for biological laboratories. The company covers an overall floorage up to 160,000㎡ and has more than 65,000㎡ of class 100,000 cleanroom. Thousands of company products such as high-end consumables of biological laboratory, reagents and laboratory instruments have been widely used in such fields as biological R&D, biological medicine, molecular diagnosis and cell therapy, command a good sale in more than 70 countries and regions such as Europe and America and are provided to world-famous colleges and universities, inspection and quarantine institutions, medical institutions, vaccine production enterprises, drug R&D enterprises and other large biomedical companies. The company owns several core technologies and advanced production processes for biological laboratory consumables and has been successfully selected into the global supply chain system for world-famous service providers of biological laboratory materials by virtue of excellent technical performance, product quality and efficient service.
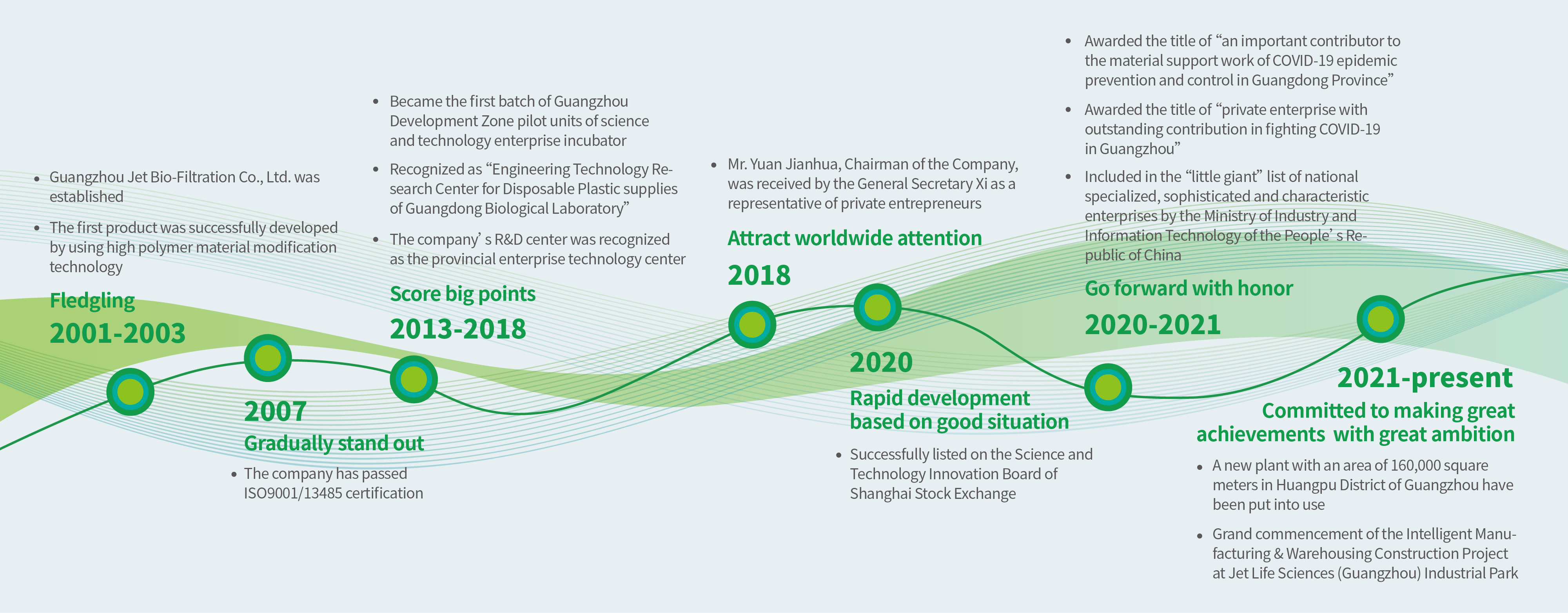
On October 24, 2018, Guangzhou Jet Bio-Filtration Co., Ltd. was honorably inspected by General Secretary Xi Jinping on behalf of private enterprises.
2001Established since
160,000m²construction area
1,200+Product SKUs
70+Countries and regions
1,600+Total emplyees+

Contribute to life science research and improve human health
Serve human health as the purpose, and become a global leading multinational enterprise in the field of life science
Provide overall solutions for life science laboratories and create maximum value for customers
Ingenuity, responsibility, carefulness and stability
Create high-quality products and services with ingenuity and responsibility and escort the life science research
Established in 2014, Guangzhou Biofil Air Purification Materials Co., Ltd., as the wholly-owned subsidiary of Guangzhou Jet Bio-Filtration Co., Ltd. (stock code: 688026)